NSE Examples#
Here we show how to find the NSE state of a set of nuclei.
Creating an NSENetwork#
import pynucastro as pyna
import numpy as np
import matplotlib.pyplot as plt
import warnings
warnings.filterwarnings('ignore')
We start by creating a Library object that reads all the ReacLib rates and link different nuclei of choice.
rl = pyna.ReacLibLibrary()
all_nuclei = ["p", "he4",
"c12", "n13",
"o16", "f17",
"ne20", "na23",
"mg24", "al27", "si28",
"p31", "s32", "cl35",
"ar36", "k39", "ca40",
"sc43", "ti44", "v47",
"cr48", "mn51",
"fe52","co55","ni56"]
lib = rl.linking_nuclei(all_nuclei)
Then initialize an NSENetwork by using the the rates created by the Library object.
nse = pyna.NSENetwork(libraries=lib, use_unreliable_spins=False)
We seek the NSE composition at a specified thermodynamic state, the NSENetwork uses scipy.optimize.fsolve() to solve the constraint equations for the proton and neutron chemical potentials. Then using this solution, it can compute the mass fractions. The main interface for this is get_comp_nse(rho, T, ye) which returns the composition at NSE as a Composition object.
Note
get_comp_nse() has options of incorporating Coulomb correction terms by setting use_coulomb_corr=True, and to return the proton and neutron chemical potentials by return_sol=True.NSENetwork also has an option of choosing to be more strict about the confidence of spin data with use_unreliable_spins=True.
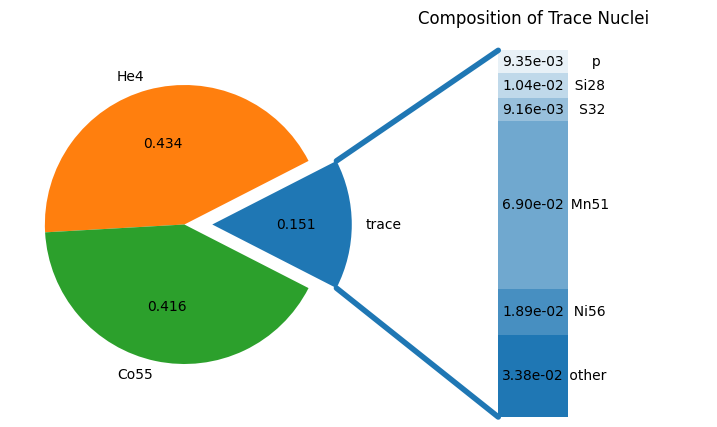
comp, sol = nse.get_comp_nse(1e7, 6e9, 0.50, use_coulomb_corr=True, return_sol=True)
fig = comp.plot()
Tip
There is pre-set initial guess for the proton and neutron chemical potentials, however one should adjust the initial guess, accordingly if no solutions are found or if the method is taking a long time to converge.
To pass in the initial guess, set initial_guess=guess, where guess is a user-supplied list of the form [mu_p, mu_n], where mu_p and mu_n are the chemical potential for proton and neutron, respectively.
We can also explicitly print the composition
for c, v in comp.X.items():
print(f"{str(c):6} : {v:15.8g}")
p : 0.0093498981
He4 : 0.43364062
C12 : 7.7285996e-06
N13 : 2.5919027e-10
O16 : 1.5697524e-05
F17 : 1.2715377e-10
Ne20 : 3.0187405e-07
Na23 : 7.2969522e-08
Mg24 : 3.4701942e-05
Al27 : 3.3525822e-05
Si28 : 0.010439163
P31 : 0.0021350073
S32 : 0.0091606019
Cl35 : 0.0040009498
Ar36 : 0.0048794488
K39 : 0.0055527548
Ca40 : 0.0047700285
Sc43 : 0.00037267165
Ti44 : 0.00022927113
V47 : 0.0049046608
Cr48 : 0.0011465823
Mn51 : 0.068958575
Fe52 : 0.0057658969
Co55 : 0.41568674
Ni56 : 0.018915105
print(f"The chemical potential for proton and neutron are {sol[0]:10.7f} and {sol[1]:10.7f}")
The chemical potential for proton and neutron are -7.7387843 and -9.9990481
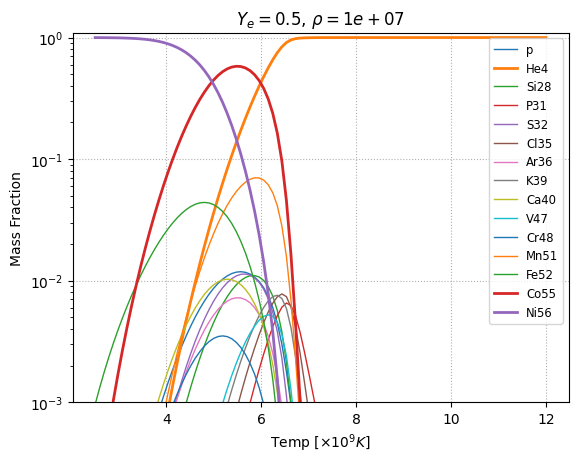
NSE variation with temperature#
For \(\rho = 10^7~\mathrm{g~cm^{-3}}\) and electron fraction, \(Y_e = 0.5\), we can explore how the NSE state changes with temperature.
Here we used a guess for chemical potential of proton and neutron of -6.0 and -11.5, respectively.
rho = 1e7
ye = 0.5
temps = np.linspace(2.5, 12.0, 100)
X_s = []
guess = [-6.0, -11.5]
for i, temp in enumerate(temps):
nse_comp, sol = nse.get_comp_nse(rho, temp*1.0e9, ye, init_guess=guess,
use_coulomb_corr=True, return_sol=True)
guess = sol
nse_X_s = [nse_comp.X[nuc] for nuc in nse_comp.X]
X_s.append(nse_X_s)
X_s = np.array(X_s)
nuc_names = nse.get_nuclei()
low_limit = 1e-3
fig, ax = plt.subplots()
for k in range(len(nuc_names)):
if np.max(X_s[:, k]) <= low_limit:
continue
lw = 2 if np.max(X_s[:, k]) > 0.1 else 1
ax.plot(temps, X_s[:, k], linewidth=lw, label=str(nuc_names[k]))
ax.legend(loc="best", fontsize="small", ncol=1)
ax.set_xlabel(r'Temp $[\times 10^9 K]$')
ax.set_ylabel('Mass Fraction')
ax.set_yscale('log')
ax.set_ylim([low_limit, 1.1])
ax.set_title(rf"$Y_e = {ye}$, $\rho = {rho:6.3g}$")
ax.grid(ls=":")
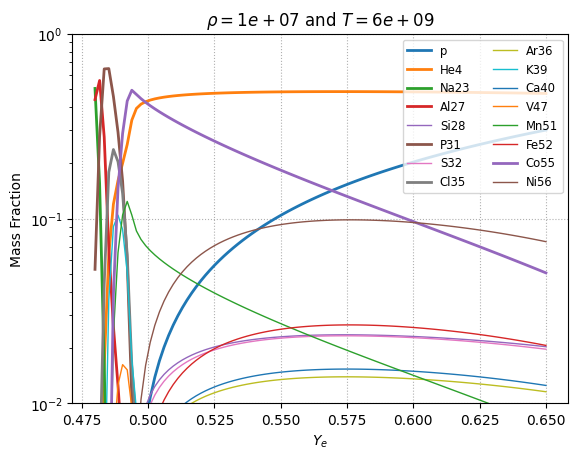
NSE variation with \(Y_e\)#
For \(\rho = 10^7~\mathrm{g~cm^{-3}}\) and a relatively high temperature of \(T = 6 \times 10^9~\mathrm{K}\), we can look at how the NSE state changes with varying \(Y_e\).
Note: there will be a lowest \(Y_e\) that is attainable by the network, depending on what species are represented. The NSE configuration with the lowest \(Y_e\) is usually difficult to find and unlikely to happen in reality. For our current example network, achieving the lowest \(Y_e\) means that mass fraction for N13 = 1. As long as protons are in the network, we can reach a \(Y_e\) of \(1\).
ye_low = min(nuc.Z/nuc.A for nuc in nse.unique_nuclei)
rho = 1e7
ye_s = np.linspace(ye_low, 0.65, 100)[1:]
temp = 6.0e9
X_s_1 = []
guess = [-6.0, -11.5]
for i, ye in enumerate(ye_s):
nse_comp_1, sol = nse.get_comp_nse(rho, temp, ye, init_guess=guess,
use_coulomb_corr=True, return_sol=True)
guess = sol
nse_X_s_1 = [nse_comp_1.X[nuc] for nuc in nse_comp_1.X]
X_s_1.append(nse_X_s_1)
X_s_1 = np.array(X_s_1)
nuc_names = nse.get_nuclei()
fig, ax = plt.subplots()
for k in range(len(nuc_names)):
if np.max(X_s_1[:, k]) <= 1e-2:
continue
lw = 2 if np.max(X_s_1[:, k]) > 0.2 else 1
ax.plot(ye_s, X_s_1[:, k], linewidth=lw, label=str(nuc_names[k]))
ax.legend(loc="best", fontsize="small", ncol=2)
ax.set_xlabel(r'$Y_e$')
ax.set_ylabel('Mass Fraction')
ax.set_yscale('log')
ax.set_ylim([1e-2, 1])
ax.set_title(rf"$\rho = {rho:6.3g}$ and $T={temp:6.3g}$")
ax.grid(ls=":")